Amyloid fibrils
Amyloid fibrils, filamentous aggregates of peptides or proteins, have been known as a cause of more than 30 neurodegenerative diseases. Understanding how each monomer unit is structured and arranged to each other in those fibrils is crucial to understand the physical basis of the fibril formation and deposition, as well as for elucidating the mechanisms of disease onset and how to prevent them.
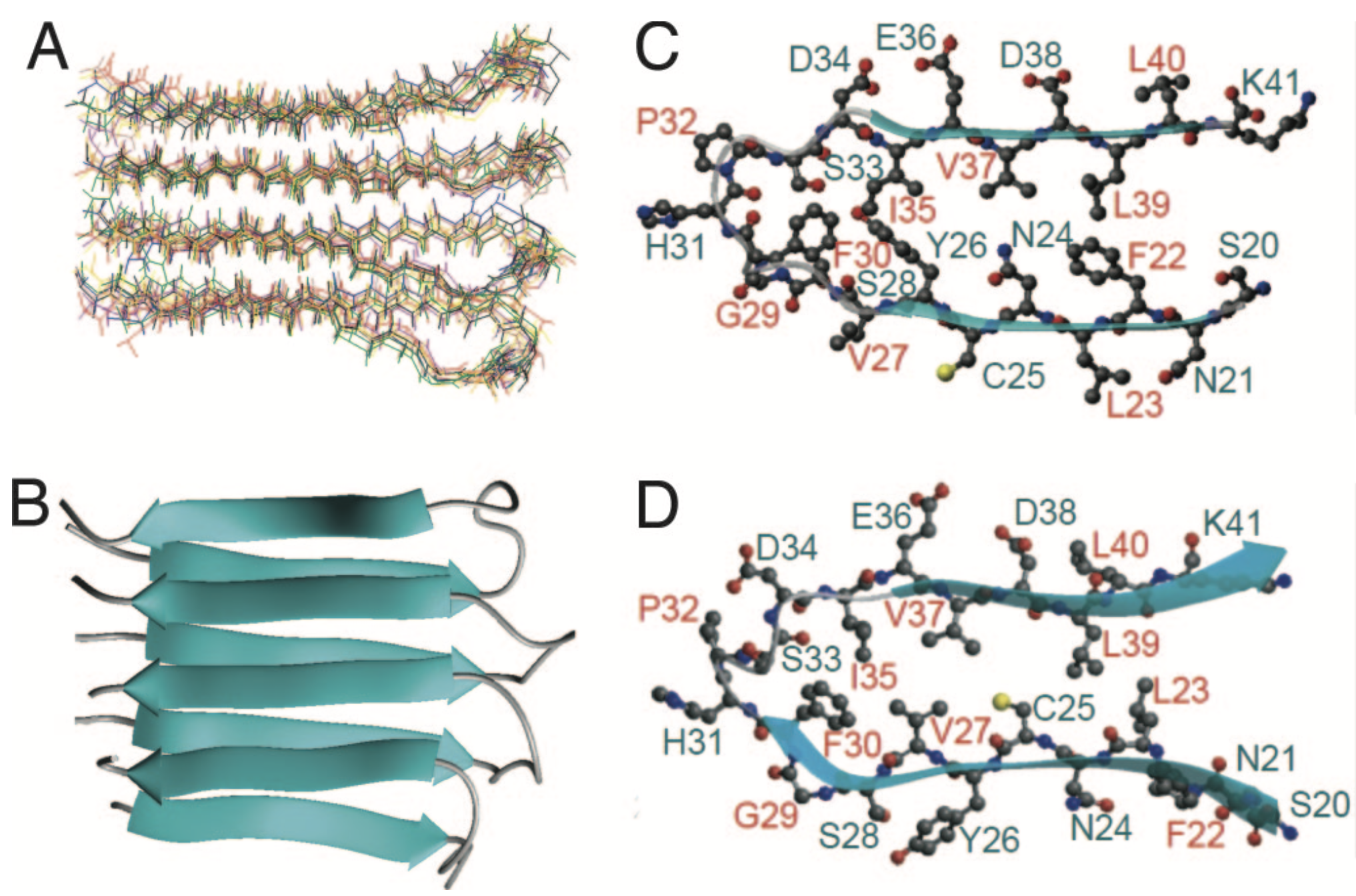
In 2006, we have combined solid-state NMR, X-ray fiber diffraction, and atomic force microscopy to reveal the 3D structure (Figure) of amyloid proto-filament-like fibrils formed by an amyloidgenic 22-residue K3 peptide (Ser20-Lys41) derived from a protein responsible for dialysis-related amyloidosis, beta2-microglobulin (beta2m), which is a part of the class I major histocompatibility complex (PNAS, 2006). As a continued exploration, we are currently working on determining structure of fibril formed by the full-length (99 residues) beta2m.
Collaboration with the University Hospital aims at directly revealing fibril structure taken from Amyloidosis patients, as well as at elucidating potential relationships between morphology of pathogenic fibrils and disease phenotypes in Parkinson disease.