Researchers find why ‘lab-made’ proteins have unusually high temperature stability
- Press Release
Bioengineers have found why proteins that are designed from scratch tend to be more tolerant to high temperatures than proteins found in nature.
Natural proteins with high thermostability are prized for their wide range of applications, from baking and paper-making to chemical production. Efforts to enhance protein thermostability—and to discover the principles behind this—are among the hottest topics in biotechnology.
The latest discoveries, published in the Proceedings of the National Academy of Sciences on November 23, 2020, open up the possibility of lab-made proteins with even better industrial applicability.
In the relatively young field of protein design, researchers have been attempting to create new types of proteins for a variety of medical, pharmaceutical, and industrial purposes. Until recently, most efforts focused on modifying natural proteins. However, such proteins are difficult to alter without disrupting their function—much like adding a fifth wheel to a car.
To overcome this limitation, some protein engineers have turned to de novo protein design: building proteins entirely from scratch. This approach, while promising, brings its own challenges, especially due to the complexity of protein folding—the process by which a protein assumes its functional shape.
In biology, structure determines function. A protein’s shape allows it to perform its role in a cell, and this shape is determined purely by physical laws. However, the interplay of these physical laws during folding remains poorly understood, making de novo design computationally difficult.
One enduring mystery in this area has been the surprisingly high thermostability of many lab-made proteins.
“For some reason, de novo proteins have repeatedly shown increased tolerance in the face of quite high temperatures compared to natural proteins,” said Prof. Nobuyasu Koga, associate professor at the Institute for Molecular Science and co-author of the study. “Where others would ‘denature’, the lab-made proteins are still working just fine well above 100 ºC.”
Design principles to date have emphasized the importance of the protein backbone—the chain of nitrogen, carbon, oxygen, and hydrogen atoms—as well as the hydrophobic core, where fatty (water-resistant) amino acids pack tightly together like puzzle pieces.
“To test which element matters more for thermostability—the backbone or the core—we modified ten amino acids involved in the core packing of our most stable de novo proteins,” explained Koga. “Surprisingly, the proteins still folded correctly and maintained high thermal stability. This suggests that the backbone structure, not core packing, plays the dominant role.”
“Hydrophobic tight core packing may not even be very important for designed proteins,” added Rie Koga, coauthor and researcher at ExCELLS. “We can create an exceptionally stable protein even if the core packing is not so optimized.”
The team now aims to refine the design principles further, especially exploring how loops and substructures within the backbone can be altered while preserving folding ability and thermostability.
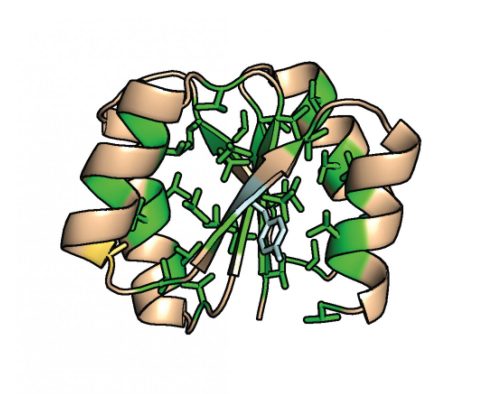
Structure of the de novo protein with most of the core filled with valine residues (green). Ten hydrophobic residues were mutated to smaller valine residues. This de novo protein still shows
high thermal stability above 100 °C.
Information of the Paper
Authors: Rie Koga, Mami Yamamoto, Takahiro Kosugi, Naohiro Kobayashi, Toshihiko Sugiki, Toshimichi Fujiwara, and Nobuyasu Koga
Journal Name: Proceedings of the National Academy of Sciences of the United States of America
Journal Title: “Robust folding of a de novo designed ideal protein even with most of the core mutated to valine”
Financial Supports
- Japan Agency for Medical Research and Development (JP19am0101072)
- Grant-in-Aid for Scientific Research (15H05592, 18K06152, 18H05420)
- Japan Science and Technology Agency (JPMJPR13AD)
Contact
Name: Nobuyasu Koga
TEL: +81-564-55-7379
E-mail: nkoga_at_ims.ac.jp
(Please replace “_at_” with @)