- Structure & Function
- Tech Developments-1
- Tech Developments-2
1. Semaphorin signaling
Semaphorins contribute to various physiological activities, including neural circuit formation, bone metabolism, angiogenesis, cancer progression, and immunity. Plexins and neuropilins are known as the major receptors for semaphorins, and each semaphorin specifically binds to a specific receptor to transmit signals. In this laboratory, we aim to elucidate how semaphorins activate their receptors and transmit their signals.
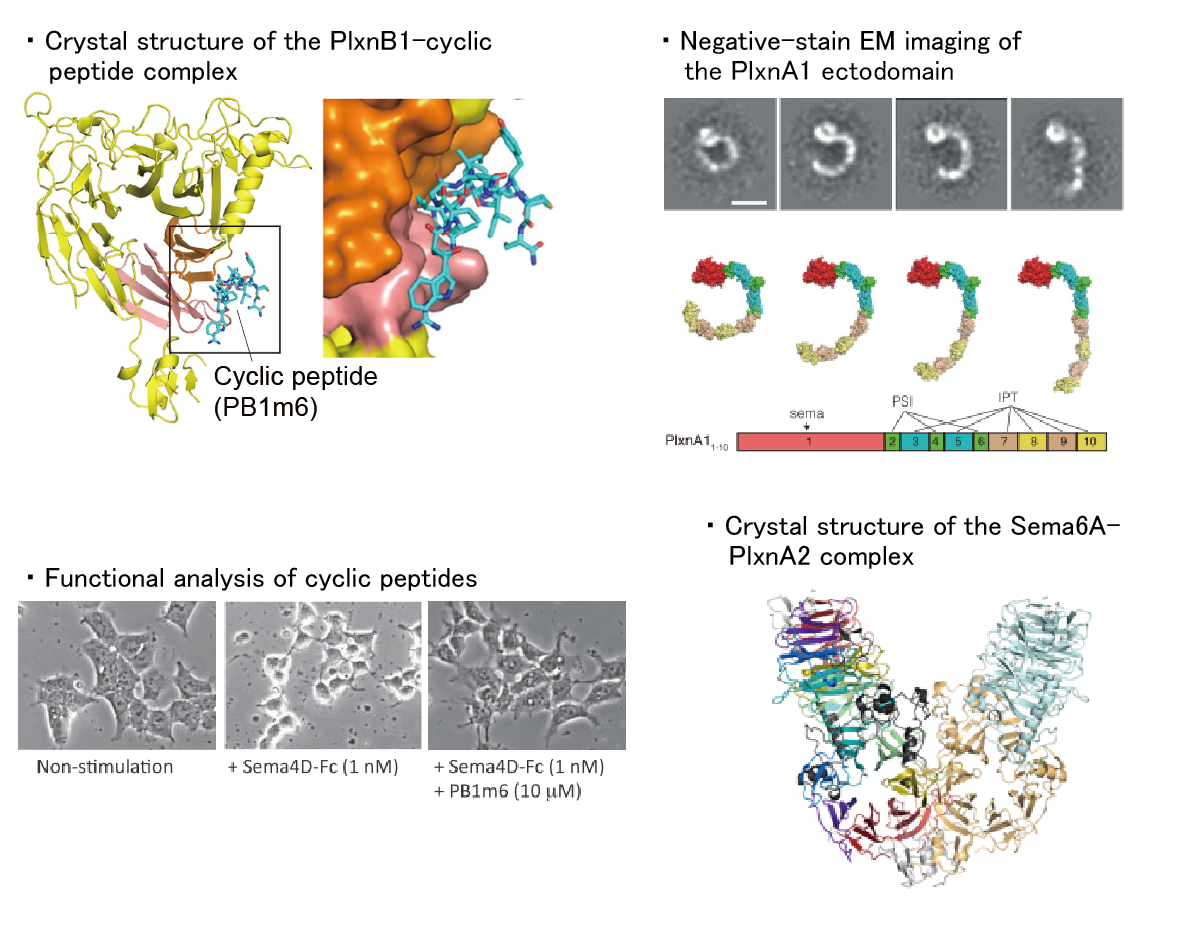
【References】
[Matsunaga Y, et al. (2016) Cell Chem. Biol.]
[Suzuki K, et al. (2016) PLoS One]
[Nogi T, et al. (2010) Nature]
2. Wnt signaling pathway
Wnt signaling pathways play important roles in the regulation of developmental processes such as cell proliferation, differentiation, and cell death. In human, 19 Wnt proteins (secreted ligands) have been identified, which activate this signaling pathway by binding to the seven-transmembrane receptor Frizzled. There are two types of Wnt signaling pathways: the canonical pathway, in which gene expression is regulated via β-catenin, and the noncanonical pathway, in which β-catenin is not required. It is also known that the canonical pathway requires a co-receptor such as LRP6 for signal transduction. Our main goal is to elucidate the structure-function relationship of the molecules involved in these signaling pathways. To this end, it is necessary to isolate the target molecules, but until now, it has been difficult to obtain purified Wnt proteins due to their ‘water-insoluble’ nature. Recently, we have succeeded in solving this ‘insoluble’ problem, and have also succeeded in determining the crystal structure of the mammalian Wnt-Frizzled complex for the first time.
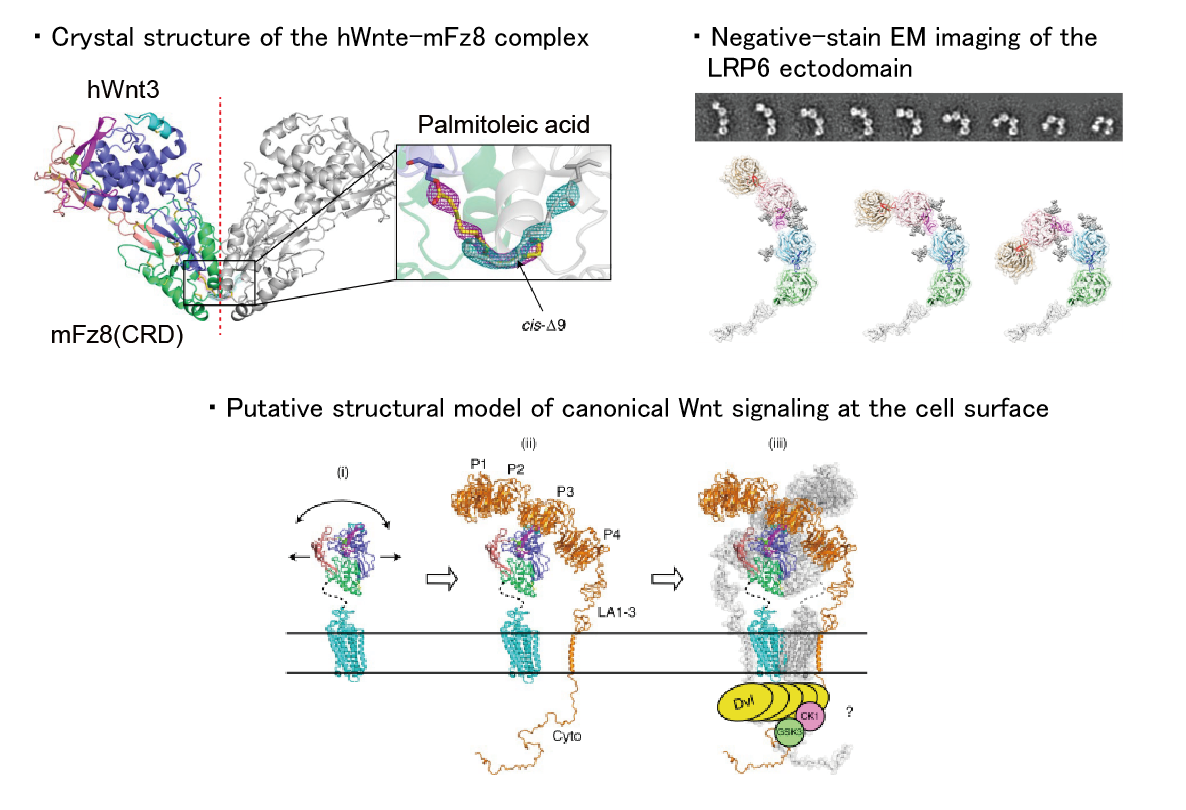
【References】
[Hirai H, et al. (2019) Nature Struct. Mol. Biol.]
[Matoba K, et al. (2017) Cell Reports]
[Mihara E, et al. (2016) eLife]
3. Structural basis for the integrin-mediated cell adhesion
Integrins are the major class of adhesion molecules that mediate cell-cell and cell-substrate adhesion. Humans have 24 integrin heterodimers each differing in their ligand binding specificity and tissue distribution. Using a collection of recombinant soluble integrin ectodomain fragments, mechanisms for the cell adhesion at molecular/atomic resolution will be elucidated.
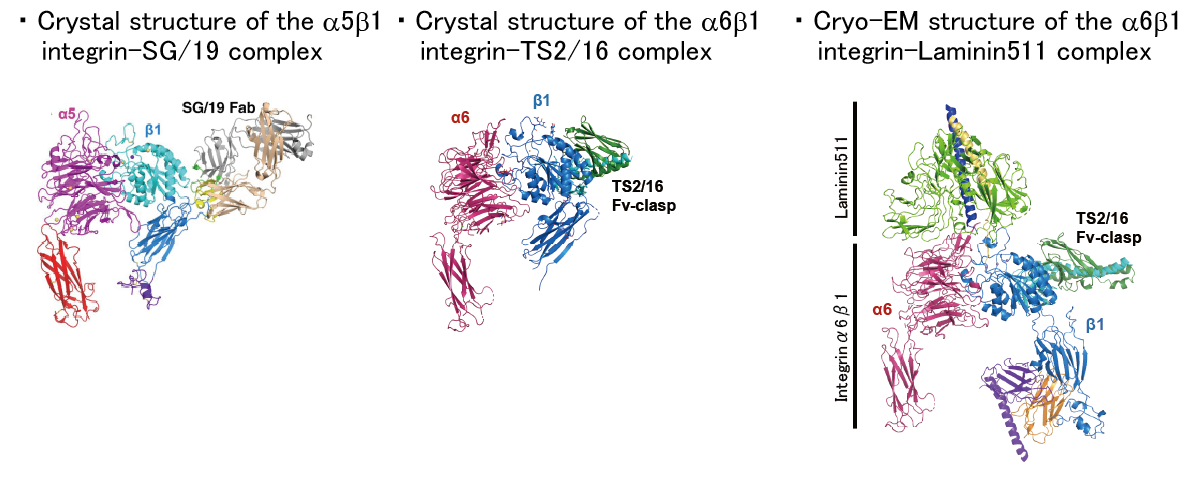
【References】
[Arimori T, et al. (2021) Nature Communications]
[Nagae M. et al. (2012) J. Cell Biol.]
4. Molecular mechanism of Met activation by HGF
HGF, a hepatocyte growth factor, is a physiological active substance that promotes organogenesis and tissue regeneration via binding to its receptor, Met. This signal is inactivated in some diseases such as liver cirrhosis, while it is over-activated in some cancers, and thus has attracted attention as a target for drug discovery. However, the molecular mechanism of Met activation by HGF is very complicated and much remains to be elucidated. We are aiming to clarify the whole picture of Met-mediated signal transduction by using structural biology approach.
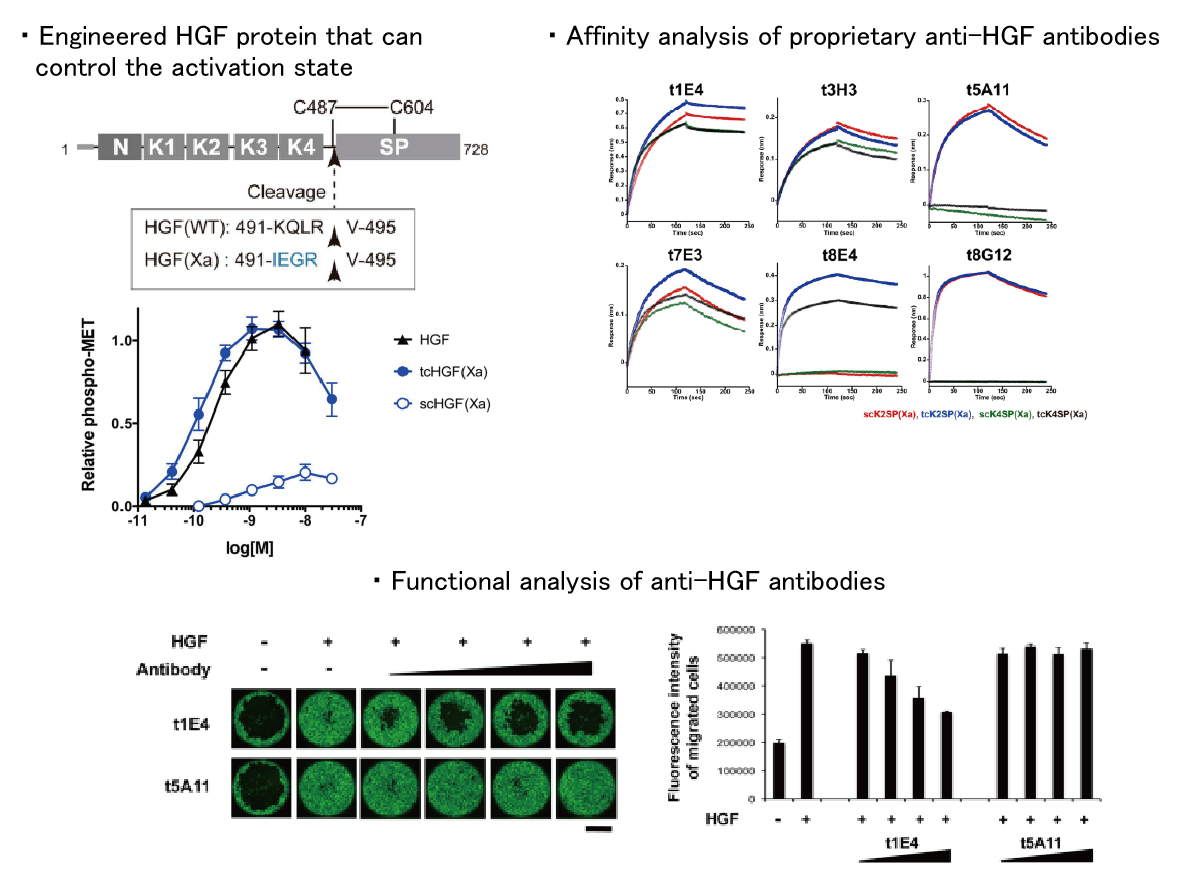
【References】
[Umitsu M, et al. (2020) J Biochem.]
[Umitsu M, et al. (2016) Sci. Rep.]
1. Development of cyclic peptide grafting technology
The RaPID system, developed by Professor Hiroaki Suga at the University of Tokyo, is capable of creating nonstandard macrocyclic peptides (consisting of ten or more residues) that bind specifically to target molecules with high affinity by using extremely diverse (in excess of 1012 compound) chemical libraries. In collaboration with the Suga Group, we have developed LassoGraft Technology®, a technology for grafting these cyclic peptides into the loop region of proteins. This technology allows us to modify various proteins, such as the Fc region of antibodies and albumin, so that they can bind to various target molecules. We are applying this technology to the creation of novel antibody-like molecules and the development of a selective gene delivery method to target cells using adeno-associated virus (AAV).
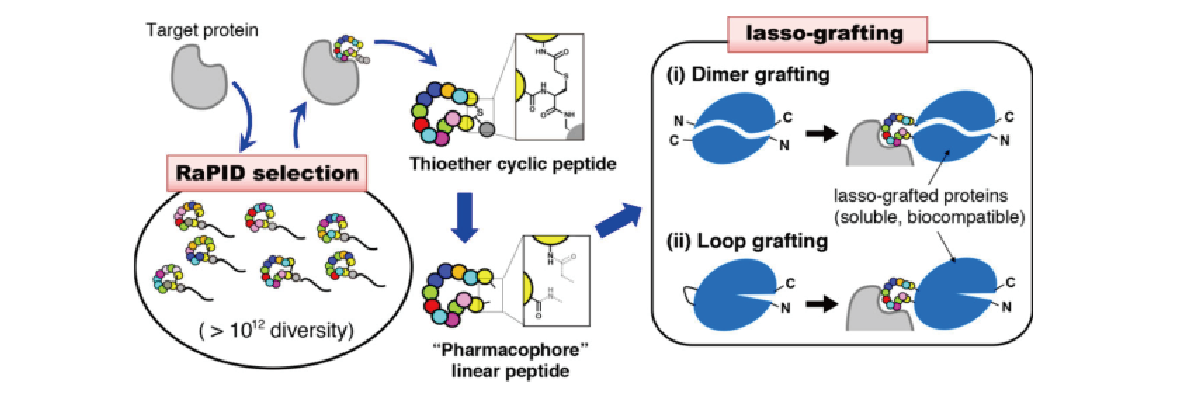
【References】
[Mihara E, et al. (2021) Nature Communications]
Novel antibody-like molecule "Mirabody®"
We have demonstrated that cyclic peptide sequences can be inserted into the loop regions of various proteins. In particular, the Fc region of antibodies is a very useful molecule as a base protein for peptide insertion. Monomeric Fc has eight peptide-insertable loops (S1-S8), and insertion of a cyclic peptide sequence into one of these loops results in a bivalent binder protein, since Fc is a homodimer. Furthermore, by inserting different cyclic peptide sequences into two or more loops, multispecific antibody-like molecules can be easily created. We have named the Fc molecules grafted with cyclic peptide(s) "Mirabody®".
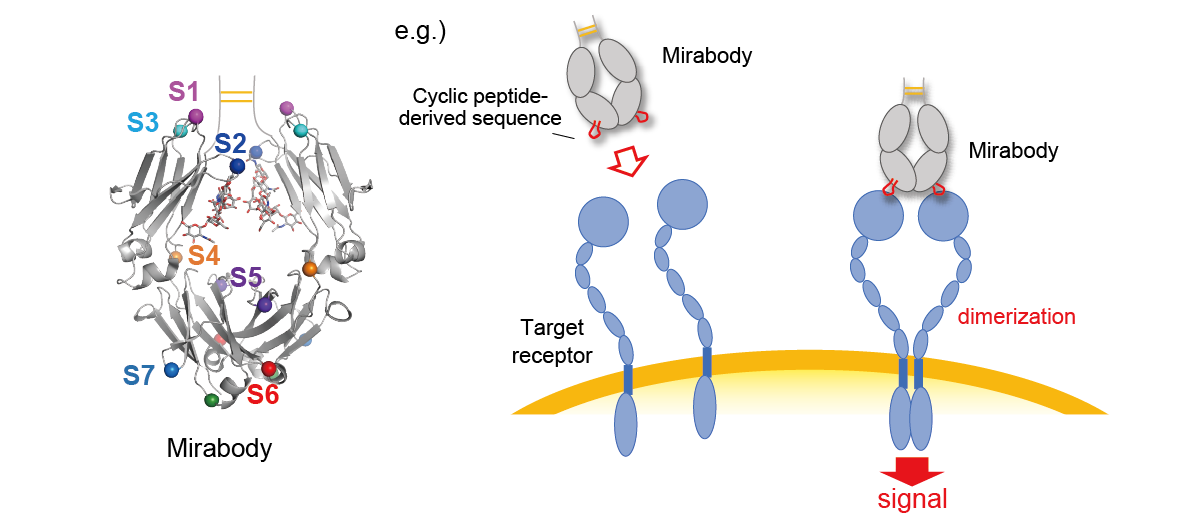
【References】
[Sugano-Nakamura N, et al. (2022) Structure]
[Skai K, et al. (2022) Nat. Biomed. Eng.]
Selective gene delivery to target cells using AAV
In the last decade, adeno-associated viruses (AAV) attained a prominent role in in vitro, in vivo, and clinical gene therapy studies given its non-pathogenicity, low immunogenicity and long-term transgene expression. However, low selectivity represents a major limitation to gene delivery with AAV vectors. In this laboratory, we use RaPID peptide insertion to surface loops of AAV capsid as an approach to improve the selectivity of the virus in infecting target tissues of interest.
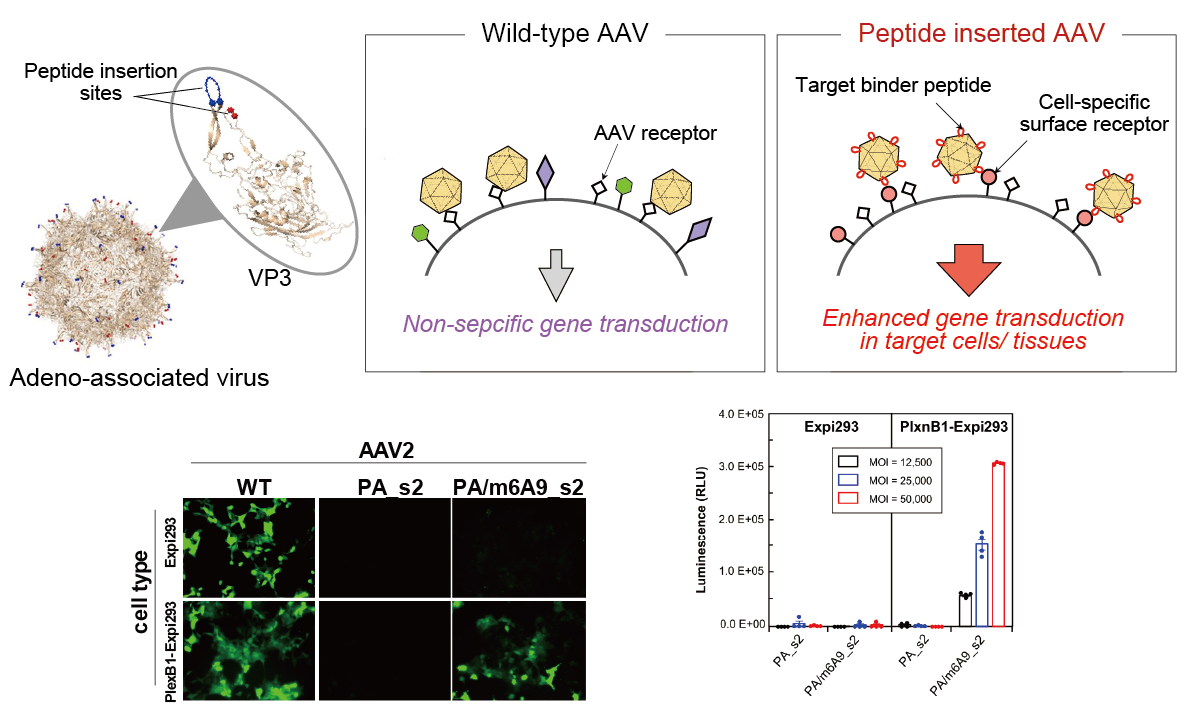
2. Small antibody fragment format "Fv-clasp"
In protein crystallization experiments, antibody fragments are used as “crystallization chaperones”, where their binding facilitates production of high-quality diffracting crystals of target molecules that are otherwise resistant to crystallization. Antibody fragments are also used for single particle analysis by cryo-EM, because they stabilize the structure of the target molecule, increase the size of the observation target, and can serve as markers for image alignment. We have developed a novel antibody fragment format “Fv-clasp” by fusing an anti-parallel coiled-coil structure derived from the human Mst1 SARAH domain with the Fv fragment of an antibody. We have demonstrated that Fv-clasp has superior properties in most if not all aspects to conventional antibody fragments including Fab and single-chain Fv. Currently, we are conducting various researches applying this Fv-clasp.
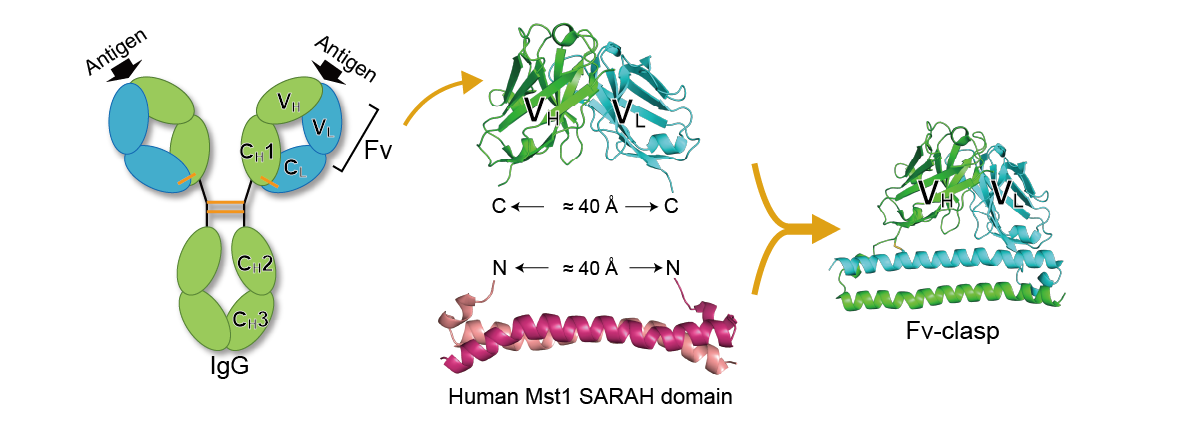
Notable features of Fv-clasp
・Compatible with bacterial expression
・Applicable to various antibodies
・High antigen binding activity
・High stability
・High crystallizability
【References】
[Arimori T, et al. (2017) Structure]
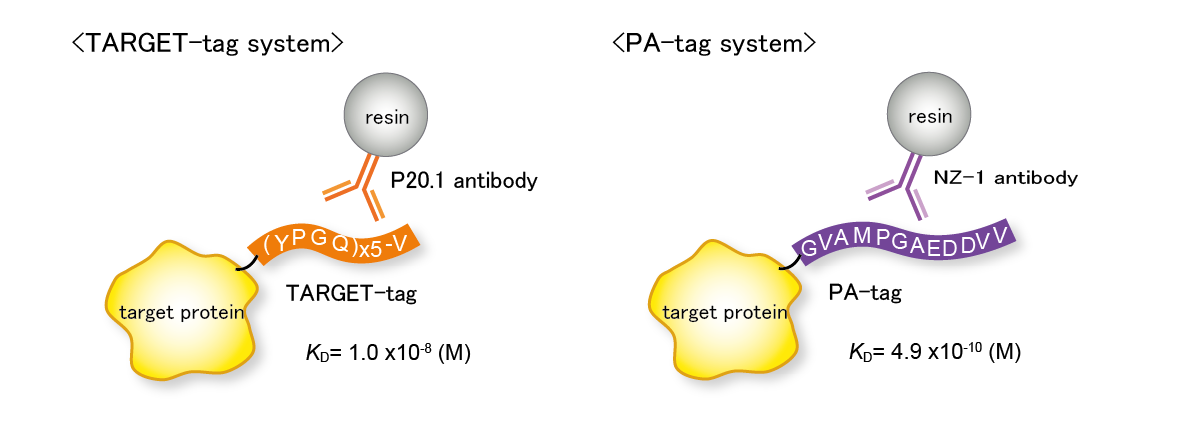
3. Novel affinity tagging system
Purification of low-concentration proteins secreted into cell culture media is problematic and demands effective affinity purification procedure. Yet commercially available affinity tagging system is expensive and does not always perform well. We are developing our own affinity tagging system using anti-peptide monoclonal antibodies or other fusion partners. To improve the performance of the tag systems, we are also working on design optimization of tag systems and development of new applications based on structural information.
TARGET-tag system / PA-tag system
・Enables one-step purification of highly purified proteins
・Elution with competitive peptides or mild solution conditions
・Resin can be used repeatedly
【References】
[Nogi T, et al. (2008) Protein Sci.]
[Fujii Y, et al. (2014) Protein Expr. Purif.]
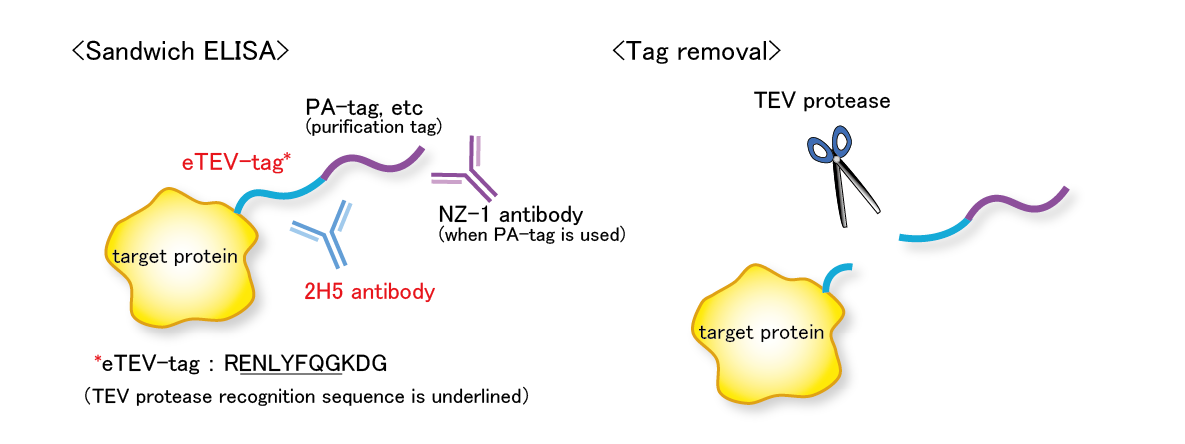
W-tag system
The eTEV tag system is a tag system that utilizes an epitope tag containing the TEV protease recognition sequence (eTEV tag) and its specific antibody 2H5. The eTEV tag is used in tandem with other tag, such as a PA tag (we call such a tandem tag system “W-tag system”). The W-tag system enables sensitive detection of target molecule using only the tag sequence without the need for specific antibodies against the target molecule. Furthermore, the tag sequence portion can be cleaved by the TEV protease.
【References】
[Tabata S, et al. (2018) Protein Expr. Purif.]
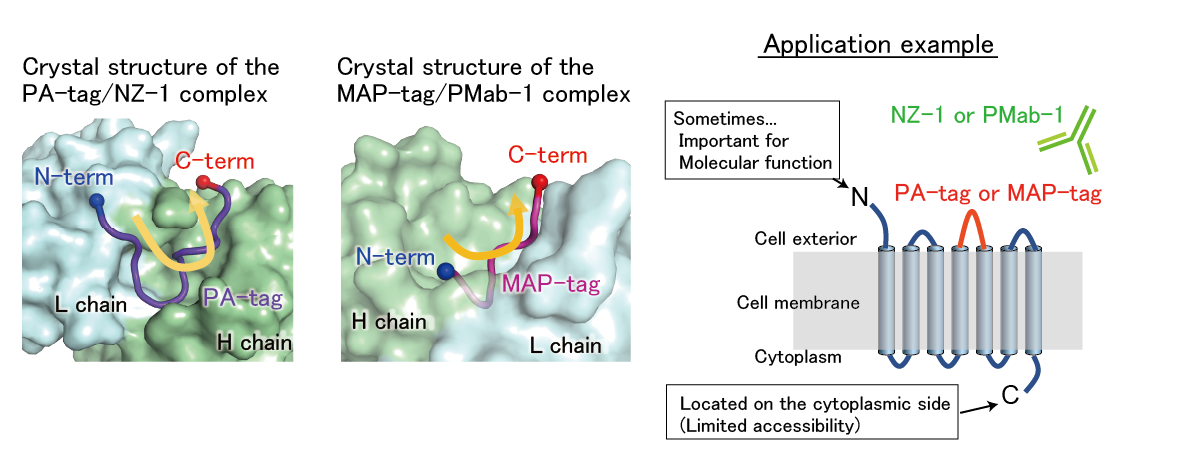
Peptide tag insertion into the loop region of a protein
The crystal structures of the PA-tag/NZ-1 and MAP-tag/PMab-1 complexes revealed that these tags bind to their respective antibodies in a U-shaped conformation where the both terminal ends are exposed to solvent. These structural features allow these tags to be inserted into the loop region of proteins without disrupting the local conformation of the target protein. By using these inserted tags, the target protein can be purified and detected in the same way as when using terminal tags.
【References】
[Fujii Y, et al. (2016) J. Cell Science]
[Wakasa A, et al. (2020) J. Biochem]
