Research
Toward understanding the molecular mechanisms underlying development
and function of the vertebrate central nervous system
〜An integrated research from genes to human diseases〜
<Research themes>
1. Molecular mechanisms regulating selective synapse formation.
2. Transcriptional and epigenetic mechanisms regulating cell fate determination in CNS development
3. Functional roles of microRNAs (miRNA) in CNS development
4. Mechanisms underlying the formation and function of cilia in the CNS and human ciliopathies
5. Generation of KO/transgenic mice and analysis of visual function of genetically modified mice
1. Molecular mechanisms regulating selective synapse formation.
While our understanding for the molecular mechanisms how neuronal axons extend to their targets has been relatively well advance, how the genetic program encoded on the genome establishes selective synapse formations among numerous neurons is still poorly understood.
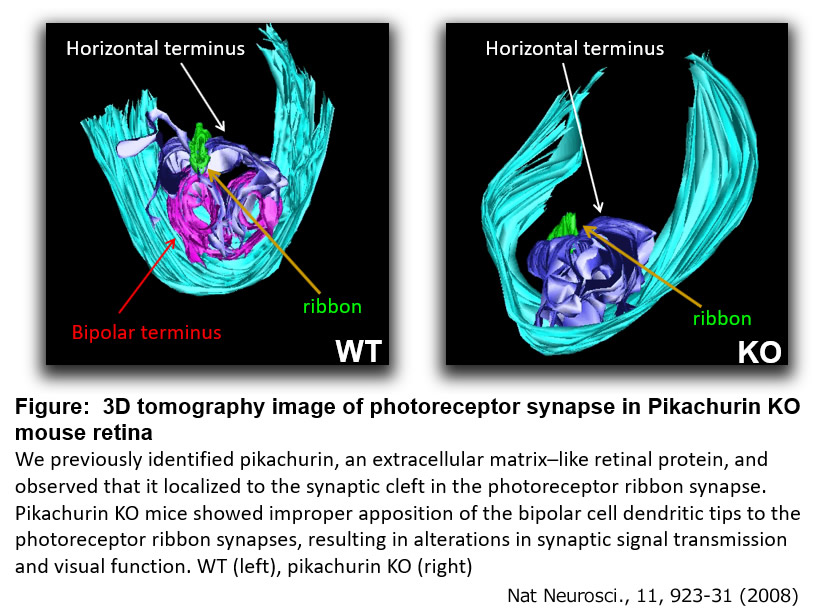
We previously identified Pikachurin as a secretory protein-encoding gene, predominantly expressed in photoreceptor cells in the mouse retina. During development, photoreceptor cell-secreted Pikachurin binds to the Dystroglycan complex at photoreceptor axon terminals and recruits GPR179 to the ON-bipolar cell dendritic terminals. This trans-synaptic complex underlies selective synapse formation and normal synaptic transmission between photoreceptor and ON-bipolar cells1-4. We identified Lrit1, an LRR cell adhesion protein, plays an essential role in selective synapse formation between cone photoreceptor cells and cone ON-bipolar cells5. We also clarified the mechanism of regulating the synapse location between photoreceptors and bipolar cells6. In addition, we identified TRPM1 as a signal transduction cation channel specifically expressed in ON bipolar cells that mediates neurotransmission between photoreceptors and ON bipolar cells and found that mutations of human TRPM1 cause congenital night blindness7-9.
Even in the retina, molecular mechanisms regulating synapse formation and circuit formation have been poorly understood. We challenge to elucidate molecular mechanisms underlying selective synapse formation by using the retina as a model system.
Research papers
1. Nat Neurosci 11(8):923-931, 2008.
2. J Biol Chem 285(41):31208-31216, 2010.
3. J Neurosci 32(18):6126-6137, 2012.
4. Cell Rep 25(1):130-145, 2018.
5. Cell Rep 2(13):3548-3561, 2018.
6. Cell Rep 10(5):796–808, 2015.
7. Proc Natl Acad Sci USA 107(1):332-337, 2010.
8. PLoS ONE 6(5):e19911, 2011.
9. J Neurosci 37(41)9889-9900, 2017.
Review
Cell Mol Life Sci. 77(7):1251-1266, 2020
We previously identified Pikachurin as a secretory protein-encoding gene, predominantly expressed in photoreceptor cells in the mouse retina. During development, photoreceptor cell-secreted Pikachurin binds to the Dystroglycan complex at photoreceptor axon terminals and recruits GPR179 to the ON-bipolar cell dendritic terminals. This trans-synaptic complex underlies selective synapse formation and normal synaptic transmission between photoreceptor and ON-bipolar cells1-4. We identified Lrit1, an LRR cell adhesion protein, plays an essential role in selective synapse formation between cone photoreceptor cells and cone ON-bipolar cells5. We also clarified the mechanism of regulating the synapse location between photoreceptors and bipolar cells6. In addition, we identified TRPM1 as a signal transduction cation channel specifically expressed in ON bipolar cells that mediates neurotransmission between photoreceptors and ON bipolar cells and found that mutations of human TRPM1 cause congenital night blindness7-9.
Even in the retina, molecular mechanisms regulating synapse formation and circuit formation have been poorly understood. We challenge to elucidate molecular mechanisms underlying selective synapse formation by using the retina as a model system.
Research papers
1. Nat Neurosci 11(8):923-931, 2008.
2. J Biol Chem 285(41):31208-31216, 2010.
3. J Neurosci 32(18):6126-6137, 2012.
4. Cell Rep 25(1):130-145, 2018.
5. Cell Rep 2(13):3548-3561, 2018.
6. Cell Rep 10(5):796–808, 2015.
7. Proc Natl Acad Sci USA 107(1):332-337, 2010.
8. PLoS ONE 6(5):e19911, 2011.
9. J Neurosci 37(41)9889-9900, 2017.
Review
Cell Mol Life Sci. 77(7):1251-1266, 2020
2. Transcriptional and epigenetic mechanisms regulating cell fate determination in CNS development
Numerous numbers of neurons reside in the brain. These extremely large number of neurons are of highly diverse. How is the information to regulate both precise identities of these neurons and formation of complicated neural networks programmed on DNA? How are cell fates of neurons and glial cells are determined during development? How extent do epigenetic mechanism and chromatin architecture contribute to this process?
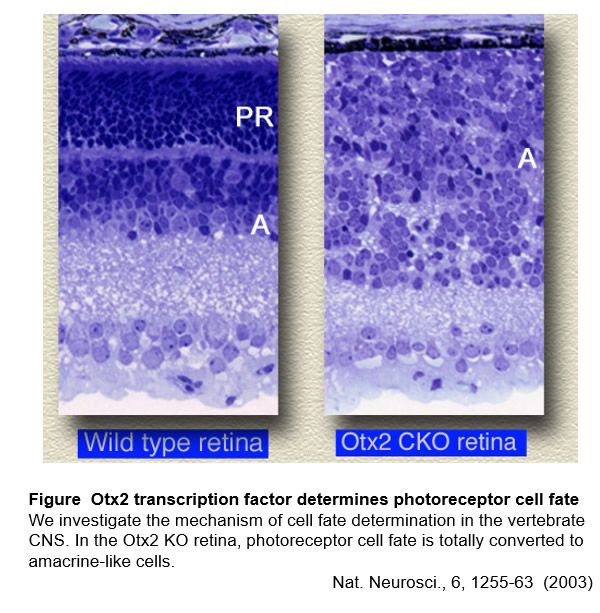
We have been studying the mechanisms of cell fate determination and cell differentiation by mainly focusing on transcriptional regulation using photoreceptor cells and other cell types in the retina as a model system. In this study, we found that Otx2 is a master transcription factor for determining photoreceptor cell fate1. We identified Crx as a master transcription factor which regulates photoreceptor maturation2-5. We also identified Rax transcription factor and elucidated its function regulating glia differentiation7 and photoreceptor maturation8. Recently, we found that epigenetic mechanisms play critical roles for establishing cell identities of retinal neurons9,10.
We have been identifying the important molecules involved in development of retinal neurons including photoreceptors. In this study, we have identified various key molecules regulating neuronal development and elucidated their mechanisms using KO mice. We aim to clarify the mechanism totally from cell fate determination to terminal differentiation at in vivo level11-14.
Research papers
1. Nat Neurosci 6(12): 1255-1263, 2003.
2. Cell 91(4):531-541, 1997.
3. Cell 91(4):543-553, 1997.
4. Nat Genet 23(4):466-470, 1999.
5. Cell Rep 30(3):658-671.e5, 2020.
6. Proc Natl Acad Sci USA 94(7):3088-3093,1997.
7. Neuron 26(2):383-394, 2000.
8. Mol Cell Biol 35(14):2583-2596, 2015.
9. Proc Natl Acad Sci USA 114 (39):E8264-E8273, 2017.
10. J Neurosci 32(49):17658-17670, 2012.
11. Mol Cell Biol 27(23):8318-8329. 2007.
12. J Neurosci 30(19):6515-6526, 2010.
13. J Neurosci 31(46):16792-16807, 2011.
14. J Neurosci 35(20):8004-8020, 2015.
We have been studying the mechanisms of cell fate determination and cell differentiation by mainly focusing on transcriptional regulation using photoreceptor cells and other cell types in the retina as a model system. In this study, we found that Otx2 is a master transcription factor for determining photoreceptor cell fate1. We identified Crx as a master transcription factor which regulates photoreceptor maturation2-5. We also identified Rax transcription factor and elucidated its function regulating glia differentiation7 and photoreceptor maturation8. Recently, we found that epigenetic mechanisms play critical roles for establishing cell identities of retinal neurons9,10.
We have been identifying the important molecules involved in development of retinal neurons including photoreceptors. In this study, we have identified various key molecules regulating neuronal development and elucidated their mechanisms using KO mice. We aim to clarify the mechanism totally from cell fate determination to terminal differentiation at in vivo level11-14.
Research papers
1. Nat Neurosci 6(12): 1255-1263, 2003.
2. Cell 91(4):531-541, 1997.
3. Cell 91(4):543-553, 1997.
4. Nat Genet 23(4):466-470, 1999.
5. Cell Rep 30(3):658-671.e5, 2020.
6. Proc Natl Acad Sci USA 94(7):3088-3093,1997.
7. Neuron 26(2):383-394, 2000.
8. Mol Cell Biol 35(14):2583-2596, 2015.
9. Proc Natl Acad Sci USA 114 (39):E8264-E8273, 2017.
10. J Neurosci 32(49):17658-17670, 2012.
11. Mol Cell Biol 27(23):8318-8329. 2007.
12. J Neurosci 30(19):6515-6526, 2010.
13. J Neurosci 31(46):16792-16807, 2011.
14. J Neurosci 35(20):8004-8020, 2015.
3. Functional roles of microRNAs (miRNAs) in CNS development
Various species express many small non-coding RNAs of 18-25 nucleotides-length, called microRNAs(miRNAs). miRNAs play various roles in development, cell differentiation, metabolism, oncogenesis, by suppressing the expression of target genes which possess the complimentary sequence of miRNAs.
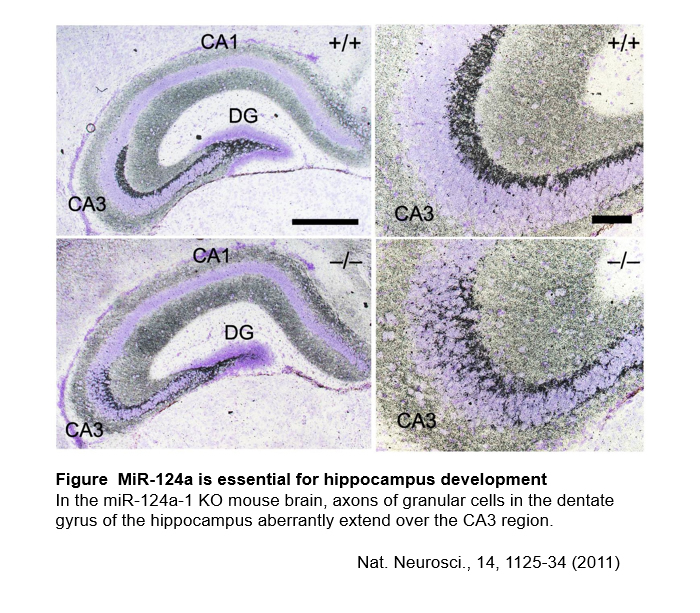
We have identified miR-124a which is predominantly expressed in the CNS and clarified its function in hippocampus development and survival of retinal cone photoreceptor cells1. We aim to advance our understanding for CNS development and function by studying functional mechanisms of miRNAs2.
Research papers
1. Nat Neurosci 14(9): 1125-1134, 2011.
2. Sci Rep 9(1):3445, 2019.
We have identified miR-124a which is predominantly expressed in the CNS and clarified its function in hippocampus development and survival of retinal cone photoreceptor cells1. We aim to advance our understanding for CNS development and function by studying functional mechanisms of miRNAs2.
Research papers
1. Nat Neurosci 14(9): 1125-1134, 2011.
2. Sci Rep 9(1):3445, 2019.
4. Mechanisms underlying the formation and function of cilia in the CNS and human ciliopathies
Cilia are evolutionally conserved microtubule-based organelles that extend from basal bodies and form on the apical surface of cells. In humans, ciliary dysfunction is associated with various diseases that can be broadly classified as “ciliopathies” , including polydactyly, brain malformation, situs inversus, obesity, diabetes, polycystic kidney, and retinal degeneration. Retinal photoreceptor cells develop a light sensory structure containing photopigments and light-transducing machinery, the outer segment. Outer segments are initially formed from the primary cilia in photoreceptor precursors. While cilia are important for our lives and activities, the molecular mechanisms underlying development, maintenance and pathology are still unclear. Development of effective therapies for ciliopathies has been awaited.
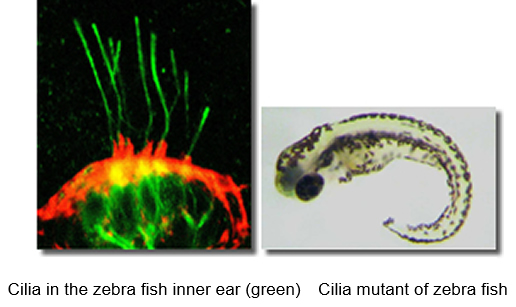
We have investigated the mechanisms of protein transport in the cilium using zebrafish1,2. We found that Mak and Ick ciliary kinases play important roles in protein transport in the cilia and cilia development using genetically modified mice3-5. Mutations of human Mak or Ick cause ciliopathies. Recently, we elucidated that Klhl18, a ubiquitin E3 ligase, regulates light-dark adaptation by modulating protein transport in rod photoreceptors6. We are studying the mechanisms of cilia development and ciliopathies using various methodologies.
Research papers
1. Nat Cell Biol 10(4):437-444,2008.
2. J Biol Chem 291(47): 24465-24474, 2016.
3. Proc Natl Acad Sci USA 107(52):22671-22676, 2010.
4. EMBO J 33(11):1227-42, 2014.
5. J Neurosci 37(8):2073-2085, 2017.
6. EMBO J 38, e101409, 2019.
We have investigated the mechanisms of protein transport in the cilium using zebrafish1,2. We found that Mak and Ick ciliary kinases play important roles in protein transport in the cilia and cilia development using genetically modified mice3-5. Mutations of human Mak or Ick cause ciliopathies. Recently, we elucidated that Klhl18, a ubiquitin E3 ligase, regulates light-dark adaptation by modulating protein transport in rod photoreceptors6. We are studying the mechanisms of cilia development and ciliopathies using various methodologies.
Research papers
1. Nat Cell Biol 10(4):437-444,2008.
2. J Biol Chem 291(47): 24465-24474, 2016.
3. Proc Natl Acad Sci USA 107(52):22671-22676, 2010.
4. EMBO J 33(11):1227-42, 2014.
5. J Neurosci 37(8):2073-2085, 2017.
6. EMBO J 38, e101409, 2019.
5. Generation of KO/transgenic mice and analysis of visual function of genetically modified mice
We generate many genetically modified mice in our laboratory, including transgenic mice, KO mice, and floxed mice. We perform various mouse bioengineering techniques, including isolation of fertilized eggs, pronucleus and blastocyst injection, mouse embryo transfer, ES cell culture, genome editing using CRISPR/Cas9 method in the lab. The genetically modified mice that we have generated are used by many researchers all over the world.
We perform visual function analysis including OKR (optokinetic response) and ERG (electroretinogram) 1-6, as well as mouse behavioral assays to understand how gene function is associated with vision and brain function in vivo7,8.
Research papers
1. Cell Rep 25(1):130-145, 2018.
2. Eur J Neurosci Feb. 6, 2020.
3. Cell Rep 10(5):796–808, 2015.
4. Sci Rep 8(1):17816, 2018.
5. Sci Rep 7(1):5540, 2017.
6. Eur J Neurosci. 38(6):2823-2831, 2013.
7. Nature Immunol 18(12):1342-1352, 2017.
8. Sci Rep 9(1):3445, 2019.
We perform visual function analysis including OKR (optokinetic response) and ERG (electroretinogram) 1-6, as well as mouse behavioral assays to understand how gene function is associated with vision and brain function in vivo7,8.
Research papers
1. Cell Rep 25(1):130-145, 2018.
2. Eur J Neurosci Feb. 6, 2020.
3. Cell Rep 10(5):796–808, 2015.
4. Sci Rep 8(1):17816, 2018.
5. Sci Rep 7(1):5540, 2017.
6. Eur J Neurosci. 38(6):2823-2831, 2013.
7. Nature Immunol 18(12):1342-1352, 2017.
8. Sci Rep 9(1):3445, 2019.
Pronucleus injection of DNA into the pronucleus of the mouse embryo to generate transgenic mice
