Research
Our current research efforts are focused on the following directions:
Comprehensive immunohistochemical localization of basement membrane proteins

The goal of our laboratory is to understand the molecular basis of tissue architecture and cellular functions in multicellular organisms based on the cell-extracellular matrix (ECM) interactions. ECM is anatomically classified into two types: the interstitial matrix and the basement membrane. We are particularly interested in the basement membrane, because the basement membrane is the prototype of ECM that is conserved throughout metazoans. We screened for additional ECM proteins by in silico computational screening for secreted proteins together with in vitro functional screening and in vivo immunohistochemical screening, and identified seven previously unknown basement membrane proteins ref.1. The immunohistochemical data was compiled into a high-resolution digital image database, which we named MOUSE BASEMENT MEMBRANE BODYMAP and is available on the internet at http://dbarchive.biosciencedbc.jp/archive/matrixome/bm/home.html.
We also analyzed the expression and localization of 20 major basement membrane proteins including all laminin chains in post-implantation mouse embryos and identified laminin-511 (α5β1γ1) as the major laminin isoform in the embryonic basement membrane underlying pluripotent epiblasts ref.2. This finding prompted us to use laminin-511 and its functionally active fragment as adhesive substrates for growing human pluripotent stem cells.
Mechanistic basis for laminin recognition by integrin
Laminin is a major basement membrane protein that regulates cell behaviors through the interaction with integrin. Laminin is a heterotrimer of three subunit chains named alpha, beta, and gamma. There are five alpha chains, three beta chains and three gamma chains in mammals, which together give rise to 16 isoforms that differ in their chain composition. Expression of these isoforms is strictly regulated in a tissue type-specific and developmental stage-dependent manners. Pluripotent stem cells in post-implantation embryos express the laminin isoforms containing the alpha5 chain, e.g., laminin-511, while mesoderm-derived cells such as skeletal and cardiac muscle cells, adipocytes, and vascular endothelial cells express the isoforms containing the alpha2 and alpha4 chains. Basal cells of stratified epithelial cells including those of skin, esophagus, and rectum express the isoforms containing the alpha3 chain, particularly laminin-332.
As is the case with laminin, there are many integrins differing in their chain composition. Integrins are heterodimeric membrane proteins composed of noncovalently associated alpha and beta subunits. There are twenty-four integrin types in mammals, of which those containing the alpha3, alpha6, and alpha7 subunits bind selectively to laminin. We recombinantly expressed all laminin binding integrins, i.e., alpha3beta1, alpha6beta1, alpha6beta4, and alpha7beta1, as truncated soluble forms, and examined their binding specificity and affinity towards a panel of laminin isoforms. Our results clearly show that the laminin-integrin interactions are primarily defined by the laminin alpha chains and the integrin alpha subunits ref.3-5).
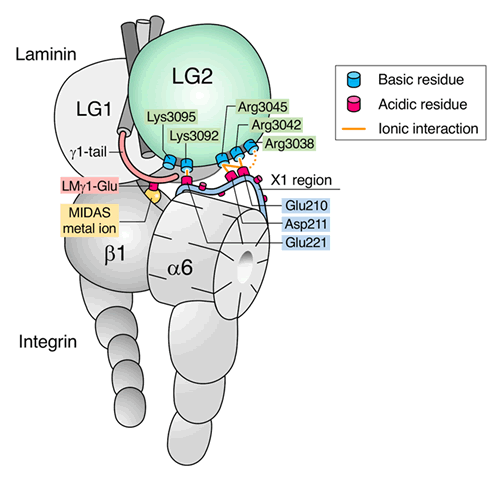
From the evolutionary viewpoint, the interaction of laminin-511 with alpha6beta1 integrin is the prototype for laminin-integrin interactions in metazoans. We succeeded in purification of laminin-511 and characterized its integrin binding specificity and affinity utilizing a truncated form of laminin-511 that fully retains its integrin binding activity. We also determined the crystal structure of the truncated form of laminin-511 and elucidated the mechanistic basis for the laminin-511 recognition by alpha6beta1 integrin by combining various biochemical techniques including exhaustive alanine scanning and intermolecular cysteine scanning assays ref.6. We found that the integrin binding site on laminin is bipartite, comprising the Glu residue in the C-terminal tail of the gamma chain and five basic amino acid residues located on the bottom face of the LG2 domain of laminin alpha5 chain. The Glu residue in the gamma tail coordinates with the divalent metal ion held in the headpiece of the integrin beta1 subunit while the five basic residues interact with a cluster of acidic residues located within the ligand recognition loop of the integrin alpha6 subunit ref.7. Our model has been confirmed by the cryo-EM structure of the complex between laminin-511 fragment and a truncated alpha6beta1 integrin ref.8(collaboration with Profs Takao Arimori and Junichi Takagi of our institute).
Designer laminin fragments for stem cell research and regenerative medicine
Cells in our body cannot survive without adhering to their surrounding ECM. This is a fundamental attribute of the cells that make up multicellular animals on the globe, known as "anchorage dependence of cell growth". Pluripotent stem cells, particularly those of humans, are strongly anchorage-dependent, and readily undergo apoptosis when they fail to adhere to culture vessels. We found that the laminin-511 E8 fragment is an excellent adhesive substrate for human pluripotent stem cells and supports their robust growth even after single cell passaging ref.9,10. The recombinant laminin-511 E8 fragment is commercially available as iMatrix-511, which is now widely used as a standard culture substrate for growing human pluripotent stem cells.
The molecular composition of ECM diversifies as development proceeds. For example, pluripotent epiblasts rely on laminin-511 in their underlying basement membrane while cells differentiated into the mesodermal lineage prefer the laminin isoforms containing alpha2 and alpha4 chains to laminin-511. We found that the efficiency of differentiation of human pluripotent stem cells into defined cell types is significantly increased by customizing the laminin isoform(s) used as an adhesive substrate. Thus, the E8 fragments of laminin-111 and laminin-411 facilitate the differentiation of human iPS cells into hepatocyte and vascular endothelial precursors ref.11,12, respectively, while the laminin-511 E8 fragment promotes the directed differentiation into dopaminergic neuronal cells as well as retinal pigmental epithelial cells ref.13.
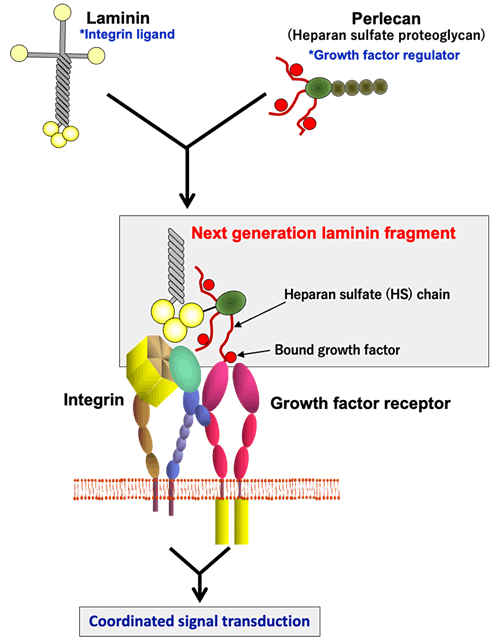
Nevertheless, there are many other cell types that cannot be induced efficiently even after customization of laminin isoforms. Cell behaviors are regulated by the signals not only transduced from ECM but also elicited by growth factors. The ECM, particularly the basement membrane, contains heparan sulfate proteoglycans that capture a variety of growth factors and modulate their availability towards their receptors on the cell surface. We produced a chimeric protein of laminin E8 fragments fused to the domain I of perlecan, to which heparan sulfate chains are attached, thereby recapitulating the synergistic signaling events elicited downstream of integrins and growth factor receptors. The resulting chimeric proteins, which we named "next generation laminin fragments", have been shown to promote the differentiation of human pluripotent stem cells to skeletal muscle stem cells and dermal stem cellsref.15.16. The next generation laminin fragments also promote the maturation of human iPS cell-derived neuronal precursor cells transplanted to the brain of the Parkinson's disease model rats ref.14.
Functions of Polydom/SVEP1 in lymphangiogenesis
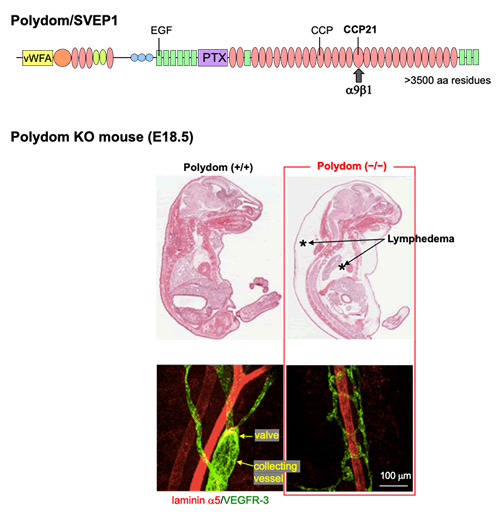
Polydom is a high-molecular-weight secreted protein that we identified by in silico screening of transcriptome and genomic databases combined with in vitro transfection and in vivo immunochemical screenings. Polydom is comprised of an array of domains characteristic of secreted proteins, including sushi, von Willebrand factor A, EGF and pentraxin domain, and also named SVEP1. We found that Polydom is a ligand for alpha9beta1 integrin and deposited in the interstitial matrices of many organs including lung, stomach, and intestine ref.17. Polydom deficient mice die immediately after birth due to respiratory failure, exhibiting severe lymphedema in the thoracic and abdominal cavities ref.18. The mice show the defects in lymphatic vessel development and fail to remodel the primitive lymphatic plexus to a single lymphatic trunk with valves, resulting in dysfunction of fluid drainage. We are currently focusing our attention on the molecular mechanism underlying the phenotype of Polydom knockout mice, particularly the involvement of Polydom in the Angiopoietin-Tie signaling system that governs lymphangiogenesis. Recently, we found that Polydom binds directly to Tie1 and promotes migration of lymphatic endothelial cells through activation of the PI3K/Akt signaling pathway ref.19. Tie1 has long been known as an orphan receptor in the Angiopoietin-Tie signaling system. Our results indicate that Polydom is the physiological Tie1 ligand that governs lymphangiogenesis as a modulator of the Angiopoietin-Tie system.